The following reaction is at equilibrium in a closed container. 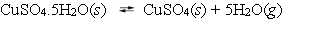 Which, if any, of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
Which, if any, of the following actions will lead to an increase in the pressure of H2O present at equilibrium?
Definitions:
Future Value
The value of a current asset or amount of money at a specified time in the future, accounting for interest or capital gains.
FV
A financial term representing the future value of an investment, accounting for factors like interest rates and time.
Quarterly Compounding
The process of calculating interest on both the initial principal and the accumulated interest over four periods within a year.
Semi-Annually
Occurring twice a year; typically referring to the frequency at which interest is paid or calculated.
Q12: Consider the equilibrium reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q25: Heats of solution may be either positive
Q29: Write the ion product expression for silver
Q32: Predict the products for the following
Q37: Which of the following conditions will
Q47: Which of the following is always
Q59: The solubility of aluminum hydroxide in water
Q68: Which of the following has the highest
Q77: The decomposition of SOCl<sub>2</sub> is first-order in
Q83: Safrole is used as a topical