When 0.152 mol of solid PH3BCl3 is introduced into a 3.0 L container at a certain temperature, 8.44 *10¯3 mol of PH3 is present at equilibrium:
PH3BCl3(s)  PH3(g) + BCl3(g)
PH3(g) + BCl3(g)
Construct a reaction table for the process, and use it to calculate Kc at this temperature. 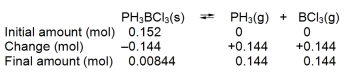
Definitions:
Advanced Practice Nurses
Registered nurses who have acquired expert knowledge base, complex decision-making skills, and clinical competencies for expanded practice.
Registered Nurses
Professional nurses who have met the required education and licensing criteria to provide patient care and support within healthcare settings.
Licensed Practical Nurses
Nurses who have obtained the necessary credentials to provide basic nursing care under the supervision of registered nurses or physicians, often involved in monitoring patients' health and providing bedside care.
Parallel Profession
Indicates a career path or profession that runs concurrently with another, often sharing similar skills or objectives but differing in specific focus areas.
Q8: For a solution equilibrium, a change in
Q13: Neon does not form any known compounds.
Q21: What will be the effect of adding
Q35: Which one of the following substances will
Q40: Write down the structure of the missing
Q41: Nitric oxide reacts with chlorine to
Q41: The decomposition of hydrogen peroxide is a
Q44: Which one of the following classes
Q90: The normal boiling point of ether is
Q99: Write the mass-action expression, Q<sub>c</sub>, for the