10.0 mL of a 0.100 mol L¯1 solution of a metal ion M2+ is mixed with 10.0 mL of a 0.100 mol l¯1 solution of a substance L. The following equilibrium is established: 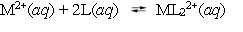 At equilibrium, the concentration of L is found to be 0.0100 mol L¯1. What is the equilibrium concentration of ML22+, in mol L¯1?
At equilibrium, the concentration of L is found to be 0.0100 mol L¯1. What is the equilibrium concentration of ML22+, in mol L¯1?
Definitions:
Unconscious
Refers to the part of the mind that influences thoughts, feelings, and behaviors without one's awareness.
Freud
Sigmund Freud was an Austrian neurologist and the founder of psychoanalysis, a clinical method for treating psychopathology through dialogue between a patient and a psychoanalyst.
Personality Inventories
Psychological assessment tools designed to evaluate a wide range of personality traits.
Projective Tests
A type of personality test in which the individual responds to ambiguous stimuli, revealing hidden emotions and internal conflicts.
Q4: Which of the following atoms has the
Q9: A) Give the names of the following
Q39: For each of the following terms/concepts, give
Q44: In the electrolysis of aqueous potassium nitrate
Q50: For a chemical reaction to be
Q65: The gas-phase conversion of 1,3-butadiene to
Q83: Amino acids in solution can undergo an
Q94: A solution is prepared by adding 0.10
Q96: Two aqueous are prepared: 1.00 m Na<sub>2</sub>CO<sub>3</sub>
Q98: Identify the organic product for the reaction