At high temperatures, carbon reacts with O2 to produce CO as follows: 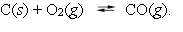 When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction?
When 0.350 mol of O2 and excess carbon were placed in a 5.00-L container and heated, the equilibrium concentration of CO was found to be 0.060 M. What is the equilibrium constant, Kc, for this reaction?
Definitions:
Weighted-Average Method
An inventory costing method that assigns a weighted average cost to each unit of inventory on hand.
Equivalent Unit
An accounting measure used to compute the number of units produced during a reporting period, converting partially completed units into an equivalent number of fully completed units.
Equivalent Units
A concept used in process costing that converts work-in-process inventory to a number of fully finished units.
Process Costing
A costing method used in manufacturing where costs are assigned to batches or production runs, typically for homogeneous products.
Q6: The nitrate anion is<br>A) a strong acid.<br>B)
Q9: There is a direct correlation between the
Q47: Identify the organic product when cyclopentanol reacts
Q49: All bimolecular reactions are second-order reactions.
Q57: What types of forces exist between molecules
Q60: Magnesium oxide, MgO, is an important industrial
Q73: A solution is prepared by adding 0.10
Q76: Nitric oxide and bromine were allowed to
Q79: Arsenic acid, H<sub>3</sub>AsO<sub>4</sub>, is used industrially to
Q88: What is the name of the acid