A mixture of 0.600 mol of bromine and 1.600 mol of iodine is placed into a rigid 1.000-L container at 350 C. 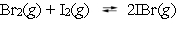 When the mixture has come to equilibrium, the concentration of iodine monobromide is 1.190 M. What is the equilibrium constant for this reaction at 350 C?
When the mixture has come to equilibrium, the concentration of iodine monobromide is 1.190 M. What is the equilibrium constant for this reaction at 350 C?
Definitions:
Occupation
Refers to one's profession or type of job, often characterized by a specific skill set or training.
Occupational Prestige
The collective evaluation by society of the desirability and importance of certain occupations over others, often based on factors such as income, education required, and perceived social value.
Social Mobility
The movement of individuals, families, or groups through a system of social hierarchy or stratification.
Social Class
Social class is a division of society based on social and economic status, influencing individuals' lifestyles, behaviors, and opportunities.
Q6: A row of the periodic table is
Q20: Benzocaine is from a family of chemicals
Q27: Which one of the following groups does
Q32: The Clausius-Clapeyron equation is used in calculations
Q32: You are given pure samples of
Q36: In order to be a Brønsted-Lowry base,
Q45: Ferric oxide is used as a pigment
Q54: Which relationship best describes <span
Q59: A gas-phase decomposition is first-order with respect
Q79: Which, if any, of the following