The following reaction, in CCl4 solvent, has been studied at 25 C. 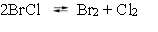
The equilibrium constant Kc is known to be 0.141. If the initial concentration of chlorine is 0.0300 M and of bromine monochloride is 0.0200 M, what is the equilibrium concentration of bromine?
Definitions:
Expert Witness
A specialist in a particular field whose opinion may help a court understand evidence or determine the fact of an issue.
Hostile Witness
A witness whose testimony is not favorable to the party calling them to testify, often showing obvious bias or reluctance.
Adverse Witness
A witness whose testimony is expected to be unfavorable to the party who has called them to testify.
Initial Client Interview
The first formal meeting between a legal professional and a prospective client to discuss the client's legal issue or case.
Q12: Consider the equilibrium reaction: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="Consider
Q20: Benzocaine is from a family of chemicals
Q27: Which of the following aqueous mixtures would
Q46: Liquid ammonia (boiling point = -33.4
Q47: In a chemical reaction, if the starting
Q62: Which element forms compounds which are involved
Q67: The colorless substance, MgF<sub>2</sub>, is used in
Q67: What is the pH of a 0.0035
Q86: The meniscus of mercury in a glass
Q102: The compound shown below is responsible for