The reaction system 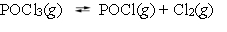 is at equilibrium. Which of the following statements describes the behavior of the system if POCl is added to the container?
is at equilibrium. Which of the following statements describes the behavior of the system if POCl is added to the container?
Definitions:
AIDS Epidemic
A global public health crisis marked by the widespread prevalence of Acquired Immunodeficiency Syndrome (AIDS), caused by the transmission of the Human Immunodeficiency Virus (HIV).
Gay Rights
The rights and protections afforded to individuals who identify as homosexual.
Traditional Aversions
Conventional disapprovals or negative attitudes towards certain behaviors, practices, or groups that deviate from societal norms.
Deviancy
A rephrased definition: Actions or behaviors that deviate from what is considered normal or acceptable by society.
Q12: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q14: Calculate the solubility of barium carbonate, BaCO<sub>3</sub>,
Q23: Salts of group 1A metals are generally
Q26: The rate law for the reaction
Q29: Secondary amines have the general formula RNH<sub>2</sub>.
Q38: Elemental boron can be formed by
Q57: What is the pH of a 0.75
Q71: A popular buffer solution consists of carbonate
Q97: Carbon tetrachloride, once widely used in
Q109: Select the correct name for this compound.