The reaction system 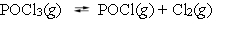 is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of chlorine is reduced by 50%?
is at equilibrium. Which of the following statements describes the behavior of the system if the partial pressure of chlorine is reduced by 50%?
Definitions:
Execution: The Discipline
Refers to the critical business practice of effectively implementing strategies and plans to achieve desired results and objectives.
Fuel Engine
A machine designed to convert the chemical energy of fuel into mechanical energy through combustion.
Leadership Effectiveness
The degree to which a leader successfully influences and guides individuals or organizations in achieving their goals.
Motives
The reasons or driving forces behind individuals' actions or behavior.
Q3: The solubility of lead(II) chloride is 0.45
Q8: You need to prepare a buffer solution
Q16: In a reversible reaction, a catalyst will
Q29: The ammonium ion, NH<sub>4</sub><sup>+</sup>, is a weak
Q42: A catalyst lowers the activation energy but
Q43: Select the correct name for this compound.
Q54: A mixture of 0.500 mole of carbon
Q63: Dinitrogen monoxide, N<sub>2</sub>O, is<br>A) a brown poisonous
Q63: A boiled egg can be cooked
Q103: Define an acid according to the Lewis