Magnesium hydroxide is used in several antacid formulations. When it is added to water it dissociates into magnesium and hydroxide ions. 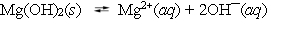 The equilibrium constant at 25 C is 8.9 *10¯12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH) 2 are now added to the mixture?
The equilibrium constant at 25 C is 8.9 *10¯12. One hundred grams of magnesium hydroxide is added to 1.00 L of water and equilibrium is established. What happens to the solution if another 10 grams of Mg(OH) 2 are now added to the mixture?
Definitions:
Hormonal Imbalance
A condition where there is too little or too much of a particular hormone in the bloodstream, affecting bodily functions.
Genetic "Map"
A representation of the location of genes or sequences on chromosomes, facilitating the study of inherited traits or diseases.
Suicide
The act of intentionally causing one's own death.
Leading Cause of Death
The primary health condition or disease that accounts for the highest number of deaths within a population or group.
Q14: When an alkali metal combines with a
Q18: Which of the following acids has the
Q24: "A diamond is forever" is one
Q26: For a chemical reaction to be
Q28: Which relationship or statement best describes
Q47: The substance NH<sub>3</sub> is considered<br>A) a weak
Q50: Which of the following compounds is ionic?<br>A)
Q83: Which of the following is the empirical
Q83: Consider the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7799/.jpg" alt="
Q98: What are the approximate carbon:hydrogen mass ratios