Methanol can be synthesized by combining carbon monoxide and hydrogen. 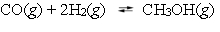 A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm. What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
A reaction vessel contains the three gases at equilibrium with a total pressure of 1.00 atm. What will happen to the partial pressure of hydrogen if enough argon is added to raise the total pressure to 1.4 atm?
Definitions:
Capacity Building
The process of developing and strengthening the skills, abilities, processes, and resources that organizations and communities need to survive, adapt, and thrive.
Social Action Strategies
Plans and methods used to address social issues and promote change for the betterment of society.
Collective Action
Activities undertaken jointly by a group of people with a common interest, often aimed at achieving a specific goal.
Community Change
Transformation within a community that affects its social, economic, environmental, or cultural conditions, often driven by collective actions or policies.
Q38: When an atom is represented by the
Q39: Most of the alkali metal salts are
Q44: Potassium nitrate is a strong reducing agent.
Q71: The acid dissociation constant K<sub>a</sub> equals 1.26
Q72: Hydrogen iodide, HI, is formed in an
Q73: The concentration of iodine in sea water
Q80: What is the pOH of a 0.0085
Q98: A solution is prepared by adding 100
Q104: Including cyclic compounds, how many possible isomers
Q108: What is the pH of a buffer