The following reaction is at equilibrium at a pressure of 1 atm, in a closed container. 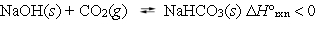 Which, if any, of the following actions will decrease the concentration of CO2 gas present at equilibrium?
Which, if any, of the following actions will decrease the concentration of CO2 gas present at equilibrium?
Definitions:
Teenage Child
An individual in the developmental stage between childhood and adulthood, typically ranging from 13 to 19 years old.
Upper-Income Family
A family that earns significantly more income than the average, placing them in the top echelons of income distribution.
Abusive Husbands
Husbands who engage in behaviors that inflict physical, emotional, psychological, or sexual harm on their partners.
Religious Pressure
The influence or coercion exerted by religious groups or beliefs on individuals or societies to conform to specific doctrines or practices.
Q3: Crystal structures may be conveniently measured using<br>A)
Q5: Which one of the following statements about
Q14: When an alkali metal combines with a
Q23: Select the weakest electrolyte from the following
Q47: Identify the organic product when cyclopentanol reacts
Q57: Identify the organic product when cyclohexanol reacts
Q57: Carbon monoxide and chlorine combine in an
Q70: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q73: Chlorine atoms act as heterogeneous catalysts in
Q102: The compound shown below is responsible for