In the gas phase at 500. C, cyclopropane reacts to form propene in a first-order reaction. The figure shows the natural logarithm of the concentration of cyclopropane (in mol/L) plotted versus time. 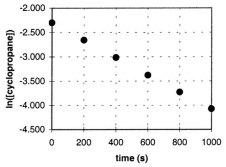 a. Explain how this plot confirms that the reaction is first order.
a. Explain how this plot confirms that the reaction is first order.
B) Calculate the first-order rate constant, k.
C) Determine the initial concentration of cyclopropane in this experiment.
Definitions:
John Barth
An American novelist known for his postmodernist and metafictional techniques, exploring the nature of narrative and the author-reader relationship.
Bildungsroman
A genre of novel that focuses on the psychological and moral growth of its main character from youth to adulthood.
Extinguished
Completely put out, terminated, or rendered non-existent, often used in the context of fires or other flames.
Healthy Life
A state of complete physical, mental, and social well-being, not merely the absence of disease or infirmity.
Q12: What is the [H<sub>3</sub>O<sup>+</sup>] in a solution
Q13: Select the correct statement about
Q22: Valence bond theory predicts that sulfur will
Q26: State the two important experimental results (and
Q51: Benzene will react with chloromethane in the
Q64: Phosphoric acid, H<sub>3</sub>PO<sub>4</sub>, is a triprotic acid,
Q75: The units of the rate of reaction
Q81: Draw and name all stable molecules with
Q84: The solubility of the oxidizing agent
Q92: All gases can be liquefied at room