Consider the general gas-phase reaction of a molecular substance, A:
1. 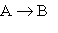 At very low pressures many such reactions occur by the following mechanism:
At very low pressures many such reactions occur by the following mechanism:
2. 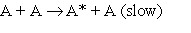 3.
3. 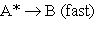 (A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
(A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
A) Which of the three reactions above is/are elementary?
B) Where appropriate, identify the molecularity of the reactions.
C) Show that the proposed mechanism is consistent with reaction 1, the observed reaction.
D) Given the mechanism above, suggest a likely rate law for reaction (1).
Definitions:
Accounts Payable
Liabilities to creditors for goods or services purchased on credit, representing obligations that a company needs to pay off in the near term.
Interest Expense
The cost incurred by an entity for borrowed funds, recognized as an expense in the income statement over the period the funds are borrowed.
Unearned Revenue
Money received by a company for services or products which have not yet been delivered or performed.
Advance Subscription Sales
Revenue received from customers for subscriptions that are paid for before the subscription period begins.
Q4: An equilibrium is established in which both
Q9: A) Give the names of the following
Q14: An increase in temperature increases the reaction
Q22: The strongest intermolecular interactions between hydrogen fluoride
Q46: Which of the following is a Lewis
Q51: The following reaction is at equilibrium at
Q55: Potassium fluoride is used for frosting glass.
Q70: Sulfur hexafluoride is a pollutant responsible for
Q78: A 2.0% (w/v) solution of sodium hydrogen
Q105: Thallium can form two oxides, Tl<sub>2</sub>O and