In the collision theory of reaction rates, the rate constant for a bimolecular reaction can be written as k = z · p · exp 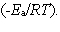 In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
In one sentence each, clearly explain the physical meaning (interpretation) of the following three factors which appear in the above expression:
A) z
B) p
C) exp 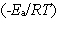
Definitions:
Custody of Assets
Responsibility for safeguarding and managing physical or digital assets, ensuring their security and proper use.
Automated Sales Systems
Technology-driven systems designed to streamline the sales process, often involving automated customer interactions, order processing, and data analysis.
Bond Employees
A bond for employees, often called fidelity bond, is an insurance policy that protects the employer against financial loss due to the fraudulent activities of an employee or group of employees.
Internal Control System
A set of policies and procedures put in place by a company to safeguard assets, ensure financial data accuracy, and promote operational efficiency.
Q6: Carbon monoxide and chlorine combine in an
Q8: If a strong acid such as HCl
Q9: Determine the freezing point of a
Q14: Given this equilibrium constant data at
Q27: Briefly outline the key arguments in the
Q29: A transition state is a species (or
Q39: Glycerin is used in cosmetics as a
Q48: Formic acid, which is a component of
Q71: For the reaction A(g) + 2B(g)
Q105: A solution of sodium acetate (CH<sub>3</sub>COONa) in