Consider the general gas-phase reaction of a molecular substance, A:
1. 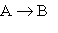 At very low pressures many such reactions occur by the following mechanism:
At very low pressures many such reactions occur by the following mechanism:
2. 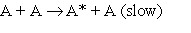 3.
3. 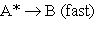 (A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
(A* represents a molecule with sufficient energy to overcome the activation energy barrier.)
A) Which of the three reactions above is/are elementary?
B) Where appropriate, identify the molecularity of the reactions.
C) Show that the proposed mechanism is consistent with reaction 1, the observed reaction.
D) Given the mechanism above, suggest a likely rate law for reaction (1).
Definitions:
J. Stacy Adams
Is known for developing the Equity Theory of motivation, which explains how individuals assess their job satisfaction and motivation through perceived fairness in the workplace.
Positive Inequity
A situation where an individual perceives they are receiving more than they believe they should in comparison to others, leading to a sense of unfair advantage.
Equity
Fairness or justice in the way people are treated, particularly regarding the distribution of resources.
Goal Setting
Is the process of setting performance targets.
Q8: For a solution equilibrium, a change in
Q9: Which of the following properties measures the
Q25: How many sigma and pi bonds, respectively,
Q35: Overlap of two sp<sup>2</sup> hybrid orbitals
Q40: Ethane can be formed by reacting acetylene
Q42: Iodine trichloride, ICl<sub>3</sub>, will react with a
Q46: Liquid ammonia (boiling point = -33.4
Q56: Which one of the following statements
Q87: Which of the following oxides will give
Q99: Serotonin transmits nerve impulses through the body.