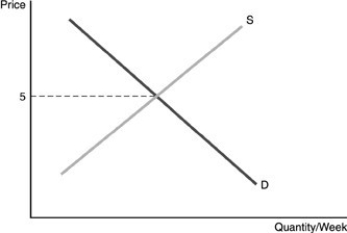
-Refer to the above figure. If an individual firm wants to maximize economic profits, it should
Definitions:
ΔG°
The change in standard Gibbs free energy during a chemical reaction, indicating spontaneity at standard conditions.
Enthalpy
A thermodynamic quantity equivalent to the total heat content of a system. It is equal to the internal energy of the system plus the product of pressure and volume.
Endothermic
Refers to a process or reaction that takes in energy from the environment, usually as heat.
Exothermic
A chemical process that releases energy to its surroundings, usually in the form of heat.
Q67: Refer to the above figure. Profits for
Q69: Refer to the above table. When the
Q85: The demand curve facing a monopolist is<br>A)downward
Q141: A monopolist's demand curve is<br>A)perfectly elastic.<br>B)perfectly inelastic.<br>C)of
Q157: When the number of substitutes increase, the
Q160: Graphically, what happens to the production function
Q202: The monopolist faces a downward sloping demand
Q233: The rate of production at which marginal
Q347: When a firm experiences declining long-run average
Q384: Refer to the above table. Given the