Identify the hybridization of atomic orbitals for atoms N1, N2, C1, C2, and C3 in caffeine, which is shown below.Explain why you think this molecule is planar or nonplanar. 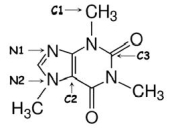
Definitions:
Simple Interest
A method of calculating the interest charge on a loan or financial product based on the original principal amount and the rate of interest over a specific time period, without compounding.
Interest on Interest
Earnings from reinvesting the interest received from an investment, leading to exponential growth over time.
Annually
Concerning an event that takes place annually.
Simple Interest
Interest calculated only on the principal amount, or on that portion of the principal amount that remains unpaid, not including interest on interest.
Q4: Which straight-chain alkane below has the highest
Q5: Which bond angle is the largest?<br>A)O -
Q25: A saturated sucrose (C<sub>12</sub>H<sub>22</sub>O<sub>11</sub>, 342.30 g/mol)
Q30: Draw the Lewis structures of PCl<sub>3</sub> and
Q54: Which statement about the following chemical
Q66: Consider the following hydrides: NH<sub>3</sub>, PH<sub>3</sub>, AsH<sub>3</sub>,
Q78: Which set of quantum numbers could
Q133: A Lewis structure of aspirin without the
Q137: How many bonding electrons are formally assigned
Q145: Which statement regarding atomic emission spectra and