Given the following reactions, what is the overall enthalpy change for the following reaction?
C4 H4 (g) + 2H2 (g) C4 H8 (g) 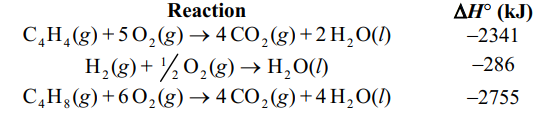
Definitions:
Sum
The total result obtained when two or more numbers or quantities are added together.
Product
An item or service created through a process, available for exchange or sale.
Pulse
The rhythmic expansion and contraction of an artery as blood is forced through it, commonly felt at various points in the body to measure the heart rate.
Symbol
An abstract or representational figure used to signify concepts, quantities, or operations in various fields such as mathematics, physics, and chemistry.
Q33: Which of the following requires the smallest
Q46: What mass of silver chloride will be
Q50: Which relationship regarding the quantities of
Q59: Which of the following is in
Q61: When 211.6 g of a waxy
Q70: Given 150 grams each of water
Q89: What is the molar mass of a
Q136: Lightweight camping stoves typically use a
Q177: Which statement below regarding water and its
Q191: If a chemical reaction causes the temperature