Given the following reactions, what is the overall enthalpy change for the following reaction?
B2 O3 (s) +6 H2 (g) B2 H6 (g) + 3H2 O(g) 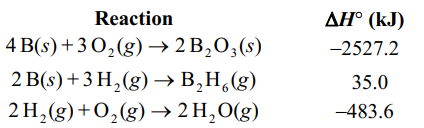
Definitions:
Cognitive Psychologist
A professional who studies mental processes such as perception, memory, thought, and problem-solving to understand human behavior and cognition.
Darwin's Theory
A scientific theory of biological evolution developed by Charles Darwin, stating that all species of organisms arise and develop through natural selection.
Determinism
The philosophical belief that all events are determined completely by previously existing causes.
Ergonomist
A professional who applies scientific information about human capabilities and limitations to the design of products, processes, and systems to enhance human safety and performance.
Q17: The concentration unit of molality is symbolized
Q22: The combustion of liquefied butane to
Q40: Perfect crystals of carbon monoxide (CO)
Q75: Paris green is a highly toxic emerald
Q118: In terms of the enthalpy of
Q121: Glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) is oxidized by molecular oxygen
Q124: Molarity, M, is defined as<br>A)moles of solute
Q149: In a steam engine, steam in a
Q196: Suppose 23.00 g ammonium chloride 53.491
Q199: Which of the following fuels has