Calculate the Enthalpy of Solution for Calcium Chloride (110 CAssume the Specific Heat of the Solution Is 4
Calculate the enthalpy of solution for calcium chloride (110.98 g/mol) using the following data: When 37.73 g of CaCl2 is dissolved in 240.00 g of water, the temperature of the solution increases by 24.25 C.Assume the specific heat of the solution is 4.18 J/(g . C).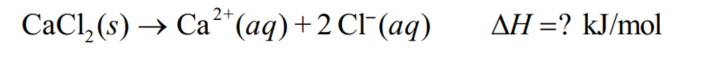
Definitions:
Secondary Market
The secondary market is where previously issued financial instruments such as stocks and bonds are bought and sold, as opposed to their initial issuance in the primary market.
Organizational Structure
The system of tasks, workflows, reporting relationships, and communication channels that link together the work of diverse individuals and groups in a coherent way.
Chief Executive Officer
The highest-ranking executive in a company, responsible for making major corporate decisions, managing the overall operations and resources of a company, and acting as the main point of communication between the board of directors and corporate operations.
Sole Proprietorship
A business owned and operated by one person, where there is no legal distinction between the owner and the business entity.
Q18: For the dimerization of nitrogen dioxide
Q30: The symbol <span class="ql-formula" data-value="\Delta"><span
Q43: How many grams of uranium hexafluoride
Q50: Give an example of a spontaneous and
Q61: Which statement below regarding heating curves is
Q70: One of the highest temperatures ever
Q95: _is how gases spread among each other;
Q104: According to the label on a bottle
Q147: The first law of thermodynamics states that<br>A)energy
Q174: What is meant by the terms solution,