Suppose 23.00 g ammonium chloride 53.491 (g/mol) dissolves in water in a calorimeter to form 100.00 g of solution.The temperature decreases by 10.75 C.Calculate the H of solution of NH4Cl in kJ/mol.Assume the specific heats of the solution and of the empty calorimeter are (4.18 J/g . C) and 0.170 kJ/ C, respectively.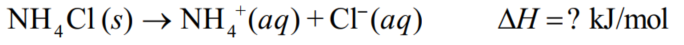
Definitions:
Interest Rate
The percentage at which interest is paid by a borrower for the use of money they borrow from a lender.
Loanable Funds
Financial resources available for borrowing, often within the context of a market setting interest rates.
Time Preference
The degree to which individuals prefer to receive goods, services, or returns on investments sooner rather than later.
Expected Inflation
The rate at which the general level of prices for goods and services is projected to rise over a specific period, influencing economic decisions.
Q16: Calculate the root-mean-square speed of an O<sub>2</sub>
Q29: Water contains about 42 mg of
Q36: At 0 K, the entropy of
Q43: A solution is made by dissolving
Q78: The kinetic energy associated with the random
Q87: Oxygen gas reacts with many metals.During this
Q117: One compound under investigation for use
Q144: To prepare 1.50 L of a solution
Q171: Use the following data to calculate the
Q198: Given that molar enthalpy of combustion