The reaction of nitrogen with oxygen to form nitrogen dioxide gas at 25 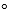 C and 1 atm requires approximately 66.4 kJ of thermal energy.The change in internal energy is about 68.9 kJ.Which statement below is true?
C and 1 atm requires approximately 66.4 kJ of thermal energy.The change in internal energy is about 68.9 kJ.Which statement below is true?
2N2 (g) +O2 (g) 2 NO2 (g)
Definitions:
Equivalent Unit
An equivalent unit is a measure used in cost accounting to express the amount of materials or labor necessary to complete a partially finished product, relative to a finished product unit.
Process Costing
A costing method used in manufacturing where costs are assigned to batches or lots of products, suitable for continuous or repetitive processes.
Conversion Costs
Conversion costs are the costs required to convert raw materials into finished products, including both direct labor costs and manufacturing overhead costs.
Equivalent Units
A concept in cost accounting used to express the amount of work done by an incomplete process on units of output, making partial units comparable to complete units.
Q7: How many grams of glucose (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>, 180.16
Q27: A gas that is produced when fruit
Q41: The normal temperature range of the
Q68: A solution is prepared by mixing
Q72: Balance the following redox reaction under
Q78: Ibuprofen is 75.69% C, 8.80% H, and
Q80: Difluoromethane (CH<sub>2</sub>F<sub>2</sub>) has a dipole moment
Q89: Air bags in cars inflate when an
Q134: The most abundant metal in Earth's crust
Q163: What is the difference between a strong