Multiple Choice
Given the following reactions, what is the overall enthalpy change for the following reaction?
C4 H4 (g) + 2H2 (g) C4 H8 (g) 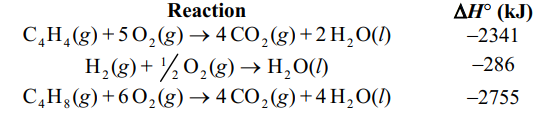
Definitions:
Related Questions
Q13: A solution contains pentane (C<sub>5</sub>H<sub>12</sub>, 72.15
Q16: What mass of nitrogen gas (28.02
Q46: What mass of silver chloride will be
Q47: Which statement regarding combustion of a sample
Q63: On a given day, the atmospheric pressure
Q65: Which of the following is in
Q95: Henry's law constant for carbon dioxide
Q102: The molar heat capacities of zinc,
Q129: Hard water, which contains Mg<sup>2+</sup> and Ca
Q134: The most abundant metal in Earth's crust