Given the following reactions, what is the overall enthalpy change for the following reaction?
B2 O3 (s) +6 H2 (g) B2 H6 (g) + 3H2 O(g) 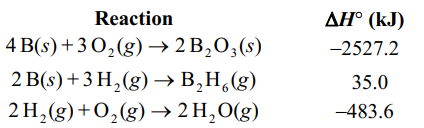
Definitions:
Symbols
Characters or glyphs that are not letters or numbers, used to represent ideas, objects, or actions.
Customized
Adapted to suit the specific needs or requirements of a user.
Account
A registered user profile on a digital platform, which provides access to services and is protected by authentication measures.
Secondary Mouse Button
The right-click button on a mouse, typically used to open context menus or provide additional interaction options.
Q10: Use the following information to determine the
Q44: Identify which of the following ionic compounds
Q57: In an experiment, 10.0 g of
Q66: Nitrogen-fixing bacteria and plants are capable of
Q71: Determine the standard entropy change of
Q75: Which of the following statements regarding the
Q88: The capacity to do work is a
Q124: What is the root-mean-square speed of a
Q135: To which of the following is the
Q202: Find the minimum amount of energy