Solved
Calculate the Enthalpy of Solution for Calcium Chloride (110 CAssume the Specific Heat of the Solution Is 4
Short Answer
Calculate the enthalpy of solution for calcium chloride (110.98 g/mol) using the following data: When 37.73 g of CaCl2 is dissolved in 240.00 g of water, the temperature of the solution increases by 24.25 C.Assume the specific heat of the solution is 4.18 J/(g . C).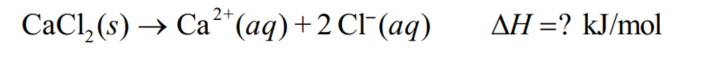
Definitions:
Related Questions
Q7: Fill in the following table regarding
Q14: N<sub>2</sub>O (laughing gas) is used as an
Q34: In a steam engine, steam in a
Q80: Which of the following regarding the vapor
Q96: Which of the following samples contains the
Q114: You make a solution of fuming nitric
Q140: Some indoor air purification systems work by
Q152: Dichlorodifluoromethane (CCl<sub>2</sub>F<sub>2</sub>, 120.91 g/mol) is a
Q157: A scientist conducts an experiment to
Q181: In a steam engine, steam in a