When magnesium reacts with hydrochloric acid, aqueous magnesium chloride and hydrogen gas are produced.Suppose 0.120 g Mg react with the HCl in a 75.000 g acidic solution contained in a calorimeter.The temperature increases by 6.03 C.Calculate the Hrxn of Mg with HCl in kJ/mol.Assume the specific heats of the solution and of the empty calorimeter are 4.18 J/g . C) and 64.4 J/ C, respectively.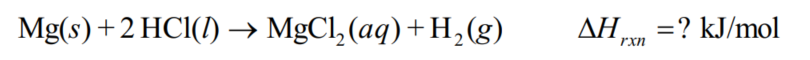
Definitions:
Uniform Commercial Code
The Uniform Commercial Code (UCC) is a comprehensive set of laws governing all commercial transactions in the United States, intended to harmonize the law of sales and other commercial transactions.
Non-negotiable Instrument
A document or contract that cannot be transferred or assigned from one person to another in a way that the receiver obtains the legal right to it.
Holder in Due Course
A party that has acquired a negotiable instrument in good faith and for consideration, thereby obtaining certain rights free of many defenses available to the original parties.
Checks
Checks are written, dated, and signed instruments that direct a bank to pay a specific amount of money from the writer's account to the person or entity in whose name the check has been issued.
Q29: Water contains about 42 mg of
Q37: What is the overall standard free
Q40: Indicate which aqueous solution has the slowest
Q49: Which statement below regarding evaporation is NOT
Q82: In a fixed-volume container at 25
Q87: What is the theoretical yield of
Q102: Write the balanced molecular equation and the
Q134: Which of the following statements regarding partial
Q156: Identify the acid in the following
Q173: One type of alcohol breathalyzer used