You wish to monitor the progress of a reaction using pressures.Suppose 17.06 g of N2 (28.02 g/mol) and 1.228 g of H2 (2.016 g/mol) are initially present in a 20.0 L reaction vessel at 127 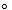 C.Nitrogen and hydrogen can react to form ammonia (17.04 g/mol) according to the reaction shown.At a given point, the total pressure (the sum of the pressures of N2, H2, and NH3 present) is 1.48 atm.What percentage of the initial N2 has reacted?
C.Nitrogen and hydrogen can react to form ammonia (17.04 g/mol) according to the reaction shown.At a given point, the total pressure (the sum of the pressures of N2, H2, and NH3 present) is 1.48 atm.What percentage of the initial N2 has reacted?
N2 (g) =3 H2 (g) 2 NH3 (g)
Definitions:
Common Stock
A type of equity security that represents ownership in a corporation, giving shareholders voting rights and a share in the company's profits through dividends.
Retained Earnings(Deficit)
The accumulated net income of a company minus any dividends paid to shareholders, reflecting the company's earnings reinvested in its operations.
Stock Split
An action by a company to increase the number of outstanding shares by dividing each share, which typically reduces the share price but does not change the overall market capitalization.
Shares Outstanding
The total number of a company's shares of stock that are currently owned by shareholders, including restricted shares.
Q9: Two solutions, A and B, are separated
Q26: A proposed mechanism for the photodecomposition
Q51: The following figure shows Arrhenius plots for
Q60: A heating curve for some substance is
Q97: Isooctane is a good model for
Q112: A mixture of cyclopropane gas (C<sub>3</sub>H<sub>6</sub>)
Q146: Three bulbs are connected as shown in
Q158: In carrying out a titration of a
Q161: Hyperbaric oxygen chambers providing pure oxygen (O<sub>2</sub>)
Q170: Briefly explain the features of a pseudo-first-order