The van der Waals constants for NO2 are a = 5.28 L2 .atm/mol2 and b = 0.04424 L/mol.What is the pressure of 56.33 g of NO2 in a 15.00 L container at 22.00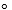 C when it is treated as
C when it is treated as
A) ideal and
B) real?
Definitions:
Transfer Payment
Money distributed by the government to individuals without any requirement for those individuals to provide goods or services in return.
Excise Taxes
Taxes imposed on specific goods, services, or transactions, typically aimed at discouraging their consumption or raising revenue for specific purposes.
Per-Unit Taxes
Taxes that are charged on a per-item basis, often levied on specific goods or services, such as gasoline or cigarettes.
Medicare & Medicaid
Federal programs in the United States that provide health coverage; Medicare serves older adults and some younger disabled individuals, while Medicaid assists low-income people.
Q18: The following figure shows Arrhenius plots for
Q31: The arrow in the diagram below indicates
Q50: In terms of the kinetic molecular theory,
Q57: A 1.80 L water sample is thought
Q64: The linear form of the Arrhenius equation
Q69: Smog created by the interaction of sunlight
Q105: Nitrogen dioxide undergoes thermal decomposition according
Q118: The equilibrium constant for the formation of
Q133: The linear form (plot of ln k
Q151: A sample of propane gas is contained