Estimate the standard molar entropy of 1.00 M aqueous Mg2+ using the following information: S for the reaction Mg(s) + 2 HCl(aq) ⇄ MgCL2 (aq) +H2 (g) is -43.02 J/K. 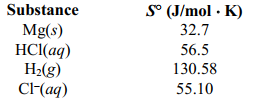
Definitions:
Statement Of Cash Flows
A financial report that shows how changes in balance sheet accounts and income affect cash and cash equivalents, breaking the analysis down to operating, investing, and financing activities.
Investing Activities
Transactions involving the acquisition or disposal of long-term assets and investments, reflected in a company's cash flow statement.
Financing Activities
This category in cash flow statements includes transactions related to raising capital and repaying investors, such as issuing stock or paying dividends.
Cash Flows
The inflow and outflow of cash and cash equivalents, representing the operating, investing, and financing activities of an entity over a period.
Q10: Use the following information to determine the
Q27: Which of the following statements regarding solubility
Q48: What pressure (in Pa) will be
Q52: The enthalpy of vaporization for bromine
Q56: In an experiment, 0.438 L of 0.152
Q59: For the reaction 3A + 2B
Q76: Which of the following statements regarding
Q83: Suppose the brain needs to metabolizes
Q97: What is the pH of a 1.3
Q128: A solution is prepared by mixing