Calculate the maximum amount of work that can be done by the reaction CH4(g) + 2 O2(g) ⇄ CO2(g) +2 H2O(g) given the following information.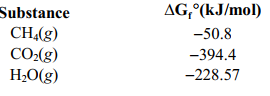
Definitions:
Profitable Growth
The process of increasing a company's revenue and profits effectively and sustainably over time.
Entrepreneurship
The act of creating, managing, and operating a business venture in order to gain profit, often characterized by risk-taking.
Act-Learn-Build
A process approach that emphasizes action-oriented learning and building iteratively on results to develop new products, services, or businesses.
Social Entrepreneurship
The pursuit of entrepreneurial ventures with a major emphasis on achieving social goals and positive externalities.
Q19: The heat of fusion for water
Q52: A solution is prepared by mixing
Q54: Which of the choices below contains
Q67: What is the device in an automobile
Q79: For the equilibrium H<sub>2</sub> (g) + S(s)
Q96: Determine the standard free energy change
Q102: Indicate which of the following has
Q109: Zinc sulfide can be oxidized by
Q110: The pressure of a gas is inversely
Q136: The linear form of the _ is