Multiple Choice
Given the following data, determine the molar free energy of combustion for liquid n-octane, C8H18. 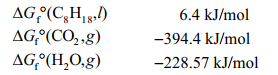
Definitions:
Related Questions
Q3: Sodium hypochlorite (NaOCl, 74.44 g/mol) is a
Q13: The observed rate law for the
Q52: What height of water in meters) is
Q56: The linear form of _ is very
Q58: Determine <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q75: Determine the molarity of an aspirin
Q108: In the Brønsted-Lowry definition of acids and
Q113: The law of mass action is a
Q113: Which statement below regarding transition states and
Q118: In terms of the enthalpy of