The change in standard molar entropy, S 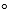 for the reaction 2 KClO3 (s) fl 2 KCl(s) +3 O2 (g) is494.0 J/mol . K at25.0011eed8be_ff85_d24e_a0ba_bddac6ebac67_TB6562_11 C.What is the standard molar entropy of O2(g) at 25.00 11eed8be_ff85_d24e_a0ba_bddac6ebac67_TB6562_11 C?
for the reaction 2 KClO3 (s) fl 2 KCl(s) +3 O2 (g) is494.0 J/mol . K at25.0011eed8be_ff85_d24e_a0ba_bddac6ebac67_TB6562_11 C.What is the standard molar entropy of O2(g) at 25.00 11eed8be_ff85_d24e_a0ba_bddac6ebac67_TB6562_11 C?
S 11eed8be_ff85_d24e_a0ba_bddac6ebac67_TB6562_11 KClO3, (s) = 143.1 J/mol .K
S 11eed8be_ff85_d24e_a0ba_bddac6ebac67_TB6562_11 KCl, (s)=82.6 J/mol . K
Definitions:
Q18: The normal boiling point of bromine
Q22: The combustion of liquefied butane to
Q24: In a spontaneous process, the entropy of
Q26: What is the boiling point of
Q31: The arrow in the diagram below indicates
Q46: A barometer measures a pressure of 745
Q51: The standard molar enthalpy and entropy
Q68: At the top of Mt.Everest, which has
Q108: In the Brønsted-Lowry definition of acids and
Q122: Which statement below regarding the difference between