Estimate the free energy change for the combustion reaction of glucose at body temperature (37 C) using the data given.If the phosphorylation of one mole of ADP3- involves a free energy change of+30.5 kJ, about how many moles of ATP4- could be made from the combustion of one mole of C6H12O6, assuming no energy loss?
C) using the data given.If the phosphorylation of one mole of ADP3- involves a free energy change of+30.5 kJ, about how many moles of ATP4- could be made from the combustion of one mole of C6H12O6, assuming no energy loss? 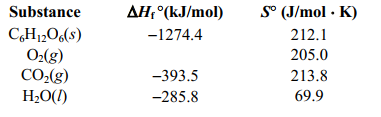
Definitions:
Top Management
The highest level of managers in an organization, responsible for setting strategic goals and making overarching decisions.
Internal Advocacy
refers to the act of promoting, supporting, or defending a cause, policy, or interest within an organization by its members.
Manages Diversity
Involves recognizing, embracing, and leveraging the differences in backgrounds, perspectives, and attributes of individuals in a group or organization to enhance its performance.
Workforce Creativity
The capacity of employees within an organization to generate innovative ideas and solutions that can improve performance and drive success.
Q1: The destruction of the ozone layer
Q5: What is the pH of a 0.65
Q47: In a spontaneous process, which of the
Q85: What is the boiling point of
Q87: An unknown gas held in a
Q89: Which statement below regarding complex ions and
Q93: Describe the effect of increasing temperature, increasing
Q97: Identify the following statement as true or
Q98: At constant T and P, any
Q125: In a catalyzed reaction, the _