Essay
For the reaction use the graph of NO2 molarity versus time in seconds to estimate the average rate of reaction over the first 400 seconds. 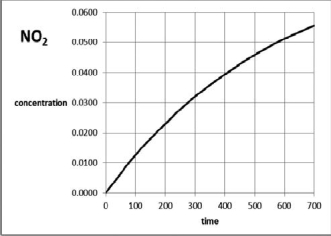
Definitions:
Related Questions
Q18: Vitamin C is a monoprotic weak acid,
Q23: The dissolution of ammonium nitrate in water
Q57: What is the pH of a 0.0175
Q61: At room temperature an increase in
Q63: Aqueous solutions of _ are acidic.<br>A)NH<sub>4</sub>Cl<br>B)Sr(NO<sub>3</sub>)<sub>2</sub><br>C)BaCl<sub>2</sub><br>D)KF<br>E)Na<sub>2</sub>CO<sub>3</sub>
Q69: You have learned that adding table
Q70: The second-order reaction A <span
Q85: The degree of ionization of a strong
Q104: A Lewis acid is<br>A)a proton donor.<br>B)a proton
Q111: Three acids found in foods are