Chlorine atoms react with methane, CH4 (g) + Cl(g) HCl(g) + CH3 (g).The rate constant for the reaction is measured at 298, 303, 308, and 313 K.
A) Using the plot of ln k vs.1/T, calculate the activation energy and the frequency factor of the reaction.
B) Estimate the rate constant at 318 K using the Arrhenius equation. 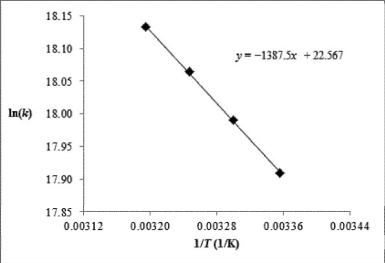
Definitions:
Cognitive Surplus
The combination of free time and the digital tools that allow people to use that time to engage in collaborative, creative activities online.
Computer Forensics
The practice of collecting, analyzing, and reporting on digital data in a way that is legally admissible, often used in the context of cybercrime investigations.
Computer Literacy
Being familiar enough with computers that a user knows how to use them and understands their capabilities and limitations.
QR Code
A machine-readable optical label that contains information about the item to which it is attached, often used for storing URLs or other data that can be read by camera-equipped smartphones.
Q22: In a catalyzed reaction, the activation
Q24: In a spontaneous process, the entropy of
Q28: For the reaction Fe(H<sub>2</sub>O)<sub>6</sub><sup>3+</sup>(aq) +H<sub>2</sub>O(l) ⇄ Fe(H<sub>2</sub>O)<sub>5</sub>(OH)<sup>2+</sup>(aq)
Q36: A second-order reaction (2 A
Q52: A proposed mechanism for the reduction
Q56: When a reduction half-reaction is written as
Q58: Which of the following pairs of liquids
Q84: How many moles of an ideal
Q91: A proposed mechanism for the reduction
Q111: What would be the freezing point