Chlorine atoms react with methane, CH4 (g) + Cl(g) HCl(g) + CH3 (g).The rate constant for the reaction is measured at 298, 303, 308, and 313 K.
A) Using the plot of ln k vs.1/T, calculate the activation energy and the frequency factor of the reaction.
B) Estimate the rate constant at 318 K using the Arrhenius equation. 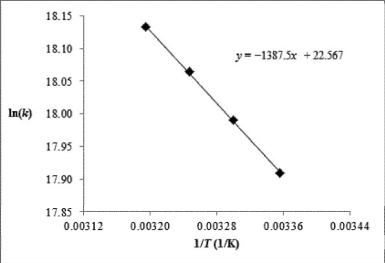
Definitions:
DNA
A molecule known as deoxyribonucleic acid, it is crucial for conveying genetic guidelines necessary for the growth, maturation, function, and reproduction of every known living organism and a wide array of viruses.
Nucleus
An organelle found in eukaryotic cells that contains most of the cell's genetic material.
Gene
Functional unit of heredity. Each gene occupies a specific place, or locus, on a chromosome; it is capable of reproducing itself exactly at each cell division; it often is capable of directing the formation of an enzyme or another protein.
Functional Unit
The smallest structure or component within an organ that is capable of performing the primary functions of the organ.
Q5: The <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q16: Suppose a 1.0 L solution containing 0.20
Q53: Codeine (C<sub>18</sub>H<sub>21</sub>NO<sub>3</sub>, 299.36 g/mol) is a weak
Q60: Estimate K<sub>p</sub> at 500 K to
Q76: Which of the following is NOT important
Q80: Which of the following regarding the vapor
Q99: The mechanism for the reaction 2
Q112: The half-life (t<sub>1/2</sub>) of a first-order reaction
Q119: Consider the following equilibrium: ClF<sub>3</sub> (g) ⇄
Q164: In an elementary step of a reaction