The observed rate law for the reaction H2 (g) + 2 ICl(g) I 2 (g) + 2 HCl(g) is first order with respect to each reactant.Draw an energy diagram that shows energy versus reaction progress for the mechanism given.Both steps are exothermic.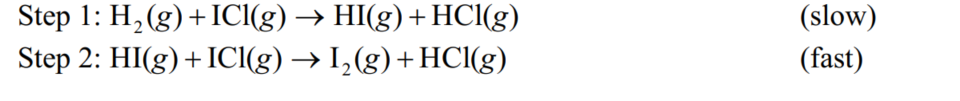
Definitions:
Important Election
A significant electoral process that has widespread implications on the governance, policy, and direction of a political body.
Lawyers
Professionals who are qualified to offer advice about the law or to represent someone in legal matters, playing a crucial role in the justice system by advocating and defending legal rights.
Civil Society
The domain of social life that is organized, voluntary, and autonomous from the state, encompassing non-governmental organizations, community groups, and other social movements.
Social Order
The set of rules, norms, and structures that govern society, maintaining stability and cohesion among its members.
Q6: Which of the following shows a relationship
Q10: The Henry's law constant for oxygen
Q14: What is the most important use for
Q21: Which statement below regarding the determination of
Q67: The reaction of bromine gas with chlorine
Q72: A solution with a pH of 9.100
Q92: Describe how you would use data obtained
Q95: Henry's law constant for carbon dioxide
Q105: A solution is prepared by adding
Q115: A scientist conducts an experiment to