You are working on a project to recycle nickel and cadmium from old nickel-cadmium batteries that have an iron casing.The batteries are dissolved in aqueous nitric acid, producing a solution containing primarily Ni2+, Cd2+, and Fe3+ cations.One idea is to add sodium hydroxide to neutralize the acid and cause precipitation of Ni(OH) 2, Cd(OH) 2, and Fe(OH) 3.Assume the concentration of each of the cations is 0.100 M before the sodium hydroxide is added.The pH increases as the sodium hydroxide is added.Which compound will precipitate first, and what is the pH at that point? 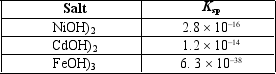
Definitions:
Operating Expenses
Costs associated with the day-to-day operations of a business, including rent, utilities, and payroll.
Sales
the exchange of goods or services for money, representing a key activity for businesses to generate revenue.
Basket Of Goods
This term refers to a fixed set of consumer products and services valued on a regular basis for the purpose of tracking inflation in an economy.
CPI
The Consumer Price Index, which measures the average change over time in the prices paid by urban consumers for a market basket of consumer goods and services.
Q19: For an equilibrium reaction with K =
Q22: What is the name of the peptide
Q28: For the reaction <span class="ql-formula"
Q28: For the reaction Fe(H<sub>2</sub>O)<sub>6</sub><sup>3+</sup>(aq) +H<sub>2</sub>O(l) ⇄ Fe(H<sub>2</sub>O)<sub>5</sub>(OH)<sup>2+</sup>(aq)
Q40: Delocalized bonding in aromatic hydrocarbons is possible
Q42: A Lewis acid is any species capable
Q49: The rate of a reaction is found
Q64: Which of the following are NOT considered
Q96: What is the hydronium ion concentration of
Q107: Which of the following statements is NOT