Based on the information in the table of standard reduction potentials below, what is the standard cell potential for an electrochemical cell that has iron Fe) and magnesium Mg) electrodes immersed in 1M Fe3+and Mg2+solutions? Also, identify the cathode. 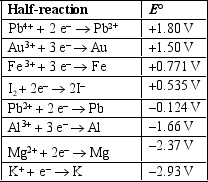
Definitions:
Destinations
The end points or locations to which something or someone is directed or being sent.
Optimal Solution
The best possible outcome or answer to a problem, derived from a set of constraints and objectives.
Transportation Model
A mathematical representation used to solve logistics problems involving the minimization of transportation costs while fulfilling supply and demand conditions.
Cells
Biological units that are considered the smallest living organisms and fundamental building blocks of life.
Q3: The <span class="ql-formula" data-value="\alpha"><span class="katex"><span
Q21: Which of the following metal hydroxides is
Q48: Which of the following statements regarding carbohydrates
Q62: Methyl methacrylate, illustrated below, is the monomer
Q80: The pH of an aqueous sodium fluoride
Q88: What is the difference between
Q118: Pure solid metals<br>A)do not crystallize.<br>B)are amorphous.<br>C)often crystallize
Q122: Using the bond enthalpy values below, the
Q134: What is the cell potential for
Q139: Which element would be used to dope