Multiple Choice
In an experiment, 100 mL of a 0.1 M solution of methionine is titrated using 0.1 M NaOH.Using this information, along with the titration curve shown, which of the following statements is NOT correct? 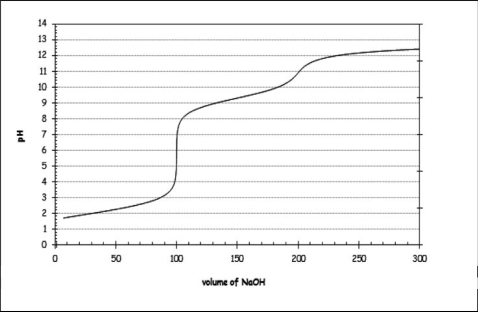
Definitions:
Related Questions
Q5: Describe what is meant by the tertiary
Q21: Region III in the nucleotide shown is
Q52: Which statement below about a cathode in
Q69: Which is the correct formula for pentaamminecyanocobalt(III)
Q85: Which d orbitals have a higher energy
Q88: A NiMH battery uses _as the reducing
Q96: Copper crystallizes in a face-centered cubic pattern.How
Q102: The greater affinity of metal ions for
Q103: Nickel-59 captures an electron to form a
Q143: In the cubic closest-packed structure of sodium