Figure 9.2
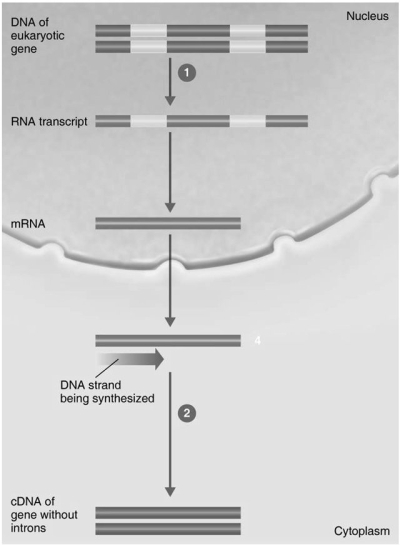
-In Figure 9.2, the enzyme in step 1 is
Definitions:
Activation Energy
The minimum amount of energy required to initiate a chemical reaction.
Endergonic
A chemical reaction in which the standard change in free energy is positive, and energy is absorbed from the surroundings.
Exergonic
Describes a reaction that ends with a net release of free energy.
Oxidized
A chemical process where a molecule, atom, or ion loses electrons, often leading to its reaction with oxygen.
Q6: In evaluating communications programs entered in PRSA
Q7: You are performing a Gram stain on
Q12: The interview format in which the person
Q20: The 'butterfly effect' describes the sensitive dependency
Q39: All of the following factors contribute to
Q44: In Figure 8.2, if base 4 is
Q45: Bacteria typically contain multiple chromosomes.
Q48: In Figure 6.3, which tube shows the
Q49: The development of DNA technology is bringing
Q55: Which microscope is used to see detail