An electron in an atom has the following set of quantum numbers:
n = 2, 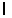 = 1,
= 1, 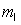 = -1, ms = +1/2.
= -1, ms = +1/2.
-In which subshell can the electron be found?
Definitions:
ICD-10-CM
The International Classification of Diseases, Tenth Revision, Clinical Modification; a system used in healthcare to classify and code diagnoses, symptoms, and procedures.
Abbreviation
A shortened form of a word or phrase used to simplify communication, often used in medical and professional contexts.
ICD-10-CM General Note
A guideline in the International Classification of Diseases, 10th Revision, Clinical Modification, providing instructions for coding and reporting medical conditions.
Inclusion Terms
Used in classifications and dictionaries to specify terms that are covered under a particular category or definition.
Q5: Which one of the following graphs shows
Q5: Two stars are just barely resolved by
Q6: In a computer monitor, electrons approach the
Q22: A convex mirror in an amusement park
Q30: The compound microscope discussed in Cutnell and
Q43: Under International Financial Reporting Standards (IFRS) the
Q48: Determine the energy of the photon emitted
Q49: Two possible states for the hydrogen atom
Q81: If point A is 16 cm below
Q102: Equity is not affected by<br>A)cash receipts.<br>B)dividends.<br>C)revenues.<br>D)expenses.