Multiple Choice
Consider the following list of electron configurations: 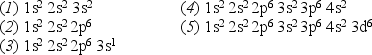
-Which one of the above configurations represents a neutral atom that readily forms a singly charged positive ion?
Understand the different directions of organizational communication (upward, downward, lateral, diagonal).
Distinguish between situations suitable for oral vs. written communication.
Recognize the importance and methods of feedback in effective communication.
Understand the role of nonverbal communication in conveying messages.
Definitions:
Related Questions
Q1: How much energy is required to remove
Q4: Income before income taxes is computed by
Q5: The income statement presents subtotals for gross
Q8: Light is incident on two slits that
Q31: Complete the following statement: The term photon
Q42: Each atom in the periodic table has
Q64: What is the rms current in the
Q68: The International Accounting Standards Board (IASB) is
Q93: Adjustments are often prepared<br>A)after the statement of
Q93: In the International Accounting Standards Board's (IASB's)