A thermally isolated sample of an ideal gas at a fixed temperature is confined to one half of a container by an impermeable membrane.The other half of the container is evacuated.The membrane is then pierced and the gas is allowed to expand freely and to double its volume as shown.Which one of the following statements is true concerning this situation? 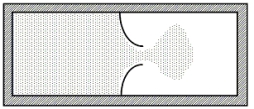
Definitions:
Current Assets
Assets that are expected to be converted into cash, sold, or consumed within one year or within the firm's operating cycle if longer.
Current Liabilities
Short-term financial obligations due within one year, including accounts payable, short-term loans, and accrued expenses.
Cash Coverage Ratio
A liquidity ratio that measures a company’s ability to pay off its debt obligations with its cash and cash equivalents.
Tax Rate
The percentage of income or value of a transaction that is required to be paid as tax to a governmental authority.
Q6: A periodic wave is produced on a
Q12: Two uncharged, conducting spheres, A and B,
Q15: A proton is traveling south as it
Q17: If a +2.0 × 10<sup>-</sup><sup>5</sup> C point
Q27: A 10-kg box is at rest at
Q32: How much work is required to move
Q32: How many antinodes are observed for the
Q43: A sealed container has a volume of
Q58: Absolute zero on the Celsius temperature scale
Q70: What is the resistance of the