Enclosed beneath the moveable piston in the drawing is 4.8 moles of a monatomic ideal gas.The gas performs work on the piston as 2300 J of heat are added from the surroundings.During the process, the temperature of the gas decreases by 45 K.How much work does the gas perform? 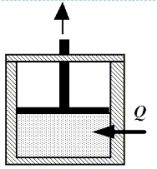
Definitions:
Equilibrium
A condition or state in which economic forces are balanced, such as when supply equals demand in a market.
Expected Total Return
The anticipated return on an investment over a given period, including both capital gains and income from dividends or interest.
Constant
A fixed value in mathematics and physics that does not change, or a situation in finance where certain conditions, like interest rate, remain unchanged over a period.
Expected Dividend
The forecasted dividend payment announced by a company for the upcoming period.
Q11: An aluminum tank of volume 0.0300 m<sup>3</sup>
Q13: What additional heat would be needed to
Q15: Two cylindrical steel rods A and B
Q20: How much energy is stored in the
Q35: Helium gas at 20 °C is confined
Q40: Complete the following statement: Bimetallic strips used
Q50: What is the total kinetic energy of
Q61: A 50-kg toboggan is coasting on level
Q64: Will the balloon rise, fall, or remain
Q77: What is the pressure of the gas