A thermally isolated sample of an ideal gas at a fixed temperature is confined to one half of a container by an impermeable membrane.The other half of the container is evacuated.The membrane is then pierced and the gas is allowed to expand freely and to double its volume as shown.Which one of the following statements is true concerning this situation? 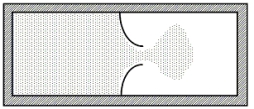
Definitions:
Supply of Tablets
Refers to the total quantity of tablet computers that producers are willing and able to sell at a given price level in a given time period.
Equilibrium Price
Equilibrium price is the price at which the quantity of a good or service demanded by consumers equals the quantity supplied by producers, resulting in market balance.
Welfare Economics
A branch of economics that focuses on the optimal allocation of resources and goods and aims to evaluate the economic well-being of individuals and society.
Total Surplus
The sum of consumer surplus and producer surplus in a market, representing the total net benefits to all participants in the market transaction.
Q1: A ceiling fan has five blades, each
Q9: The coefficient of linear expansion of steel
Q14: Determine the mass of the block.<br>A)1.02 kg<br>B)2.02
Q16: A single circular loop of wire of
Q34: Complete the following statement: The magnitude of
Q36: A satellite follows a circular path with
Q42: Three resistors and two batteries are connected
Q63: Which one of the following circuits has
Q66: Two golf carts have horns that emit
Q66: A tanker ship is filled with 2.25