An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
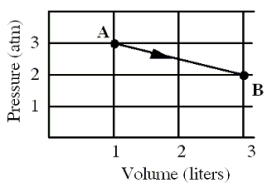
-What is the change in the internal energy,in calories,of the gas for this process?
Definitions:
Epigastric Pain
Discomfort or pain located in the upper abdomen, typically above the stomach.
Blood Glucose Levels
The concentration of sugar in the blood, which is a critical metric in managing diabetes.
Respiratory Rate
The number of breaths a person takes per minute and is a vital sign used to assess the patient's respiratory health.
Problem-focused Coping
A strategy that involves directly addressing a stressor or problem to effectively manage or solve it.
Q4: Two engines are identical except that engine
Q4: The decibel level of a jackhammer is
Q9: What is the period of the induced
Q11: What percentage of the power produced at
Q13: During the time a compact disc (CD)
Q17: If a +2.0 × 10<sup>-</sup><sup>5</sup> C point
Q26: A string is wrapped around a pulley
Q31: A simple pendulum on earth has a
Q44: At what speed is mercury flowing past
Q63: During a typical workday (eight hours), the