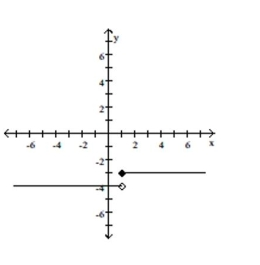
Give a rule for the piecewise-defined function. Then give the domain and range.
-
Definitions:
AntiAromatic
Compounds that have conjugated pi electron systems with 4n pi electrons, which destabilize the molecule, making it less stable than if the pi electrons were localized.
NonAromatic
Refers to organic compounds that do not possess the special stability associated with cyclic, planar systems that follow Huckel’s rule of delocalized electrons.
π Network
A system of conjugated pi bonds within a molecule that allows for the delocalization of pi electrons across all the adjacent aligned p orbitals.
Extremely Slow Rate
Describes a process or reaction that proceeds at a very slow pace, often due to kinetic barriers or unfavorable conditions.
Q24: <span class="ql-formula" data-value="\text { How can the
Q84: Suppose a cost-benefit model is given
Q143: An 8-sided die is rolled. The
Q197: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7514/.jpg" alt=" A)
Q228: The surface area a of a
Q309: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB7514/.jpg" alt=" A) Not a
Q320: <span class="ql-formula" data-value="\begin{array}{l}\text { Student Test Score
Q353: <span class="ql-formula" data-value="\text { Plot the point
Q425: A faucet is used to add
Q475: Why does a function defined by a