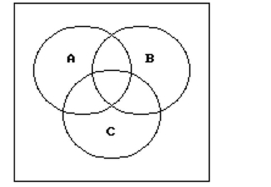
Shade the regions representing the set.
- 
Definitions:
Dalton's Law
The principle that the total pressure exerted by a mixture of gases is equal to the sum of the pressures that each would exert if it were present alone.
Partial Pressures
The pressure that a single gas in a mixture of gases would exert if it alone occupied the entire volume of the mixture.
Total Pressure
The combined pressure exerted by a mixture of gases in a container, equal to the sum of the pressures that each gas would exert if it were alone.
Gases
States of matter consisting of particles that have neither a defined volume nor shape, able to expand freely to fill any space available, such as oxygen and nitrogen.
Q11: {a, b, c, d} and {A, B,
Q30: A = 143°<br>A)53°<br>B)63°<br>C)47°<br>D)37°
Q34: Computer Specialists is planning a group
Q45: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5007/.jpg" alt=" A)
Q81: <span class="ql-formula" data-value="x \geq - 50"><span class="katex"><span
Q101: <span class="ql-formula" data-value="\{ x \mid x"><span class="katex"><span
Q120: <span class="ql-formula" data-value="\sim ( \sim p \wedge
Q169: <span class="ql-formula" data-value="\left( \mathrm { B }
Q308: <span class="ql-formula" data-value="( A \cap B )
Q311: <span class="ql-formula" data-value="\{ x \mid x"><span class="katex"><span