Solve the problem.
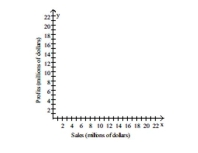
-The table below shows the sales and profits of a company from 2000 to 2005. Construct a scatter diagram for the data and state whether sales and profits for this company have no correlation, a positive correlation, or a negative correlation for this period.
Definitions:
Entropy Term
A thermodynamic quantity representing the degree of disorder or randomness in a system, significant in predicting the spontaneity of processes.
ΔG°
Standard Gibbs free energy change, a thermodynamic quantity indicating the spontaneity of a reaction at standard conditions; negative values suggest spontaneity.
Exothermic
A chemical reaction or process that releases heat to its surroundings.
Endothermic
Describes a process or reaction that absorbs energy from its surroundings, typically in the form of heat.
Q14: When 15 gallons of gasoline are
Q16: The heights of a large population of
Q22: Suppose you know that the distribution of
Q26: In 1990, the average math SAT
Q33: a. The occurrence of the digits 0,
Q35: Consider the selection of a nominating committee
Q46: A person's political affiliation<br>A)Qualitative because it is
Q51: A manufacturing process has a 70% yield,
Q93: A researcher wishes to estimate the proportion
Q128: A monthly electric bill (in dollars)<br>A)Quantitative because